Proprietary Electrocompaction Technology
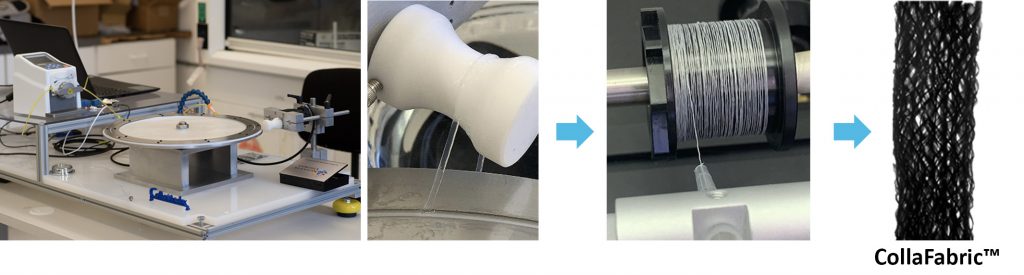
CollaMedix’s patented electrocompaction (ELAC) fabrication process enables manufacturing of collagen biotextiles by converting solubilized medical-grade collagen from commercial suppliers into threads that are among the densest and strongest forms of reconstituted collagen. These robust pure collagen threads are strong enough to use in standard textile manufacturing processes including knitting, weaving, filament winding, and braiding. CollaMedix manufactures a macroporous pure collagen biofabric: CollaFabric™.



Mimics Native Collagen
While native collagen is highly aligned and densely packed, the collagen that is reconstituted in commercial laboratories have characteristics of random orientation and loose molecular packing. Therefore, most collagen products are in the form of sponges or pellets that are used in non-load bearing applications such hemostatic or dermal fillers. The present electrocompaction process is among the most successful of collagen processing modalities. The process elevates collagen tensile strength by orders of magnitude through compaction and unidirectional alignment of collagen molecules forming novel pure collagen threads.
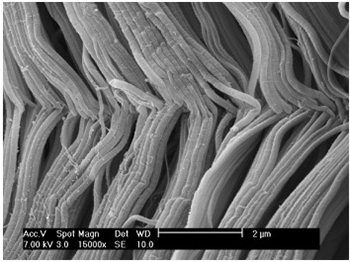
Nature:
– Uniformly oriented
– Densely packed
– Failure strength approaches bone
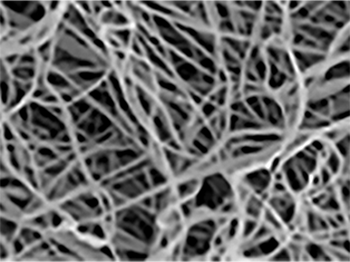
Man-made analogs/Competitors:
– Randomly oriented
– Loosely packed
– As strong as JELL-O
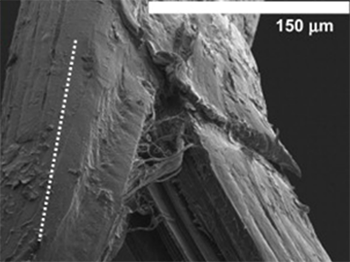
CollaMedix’s ELAC CollaFabric®:
– Aligns better
– Densely packed throughout
– Crosslinked better
Unique Bioinductive Properties
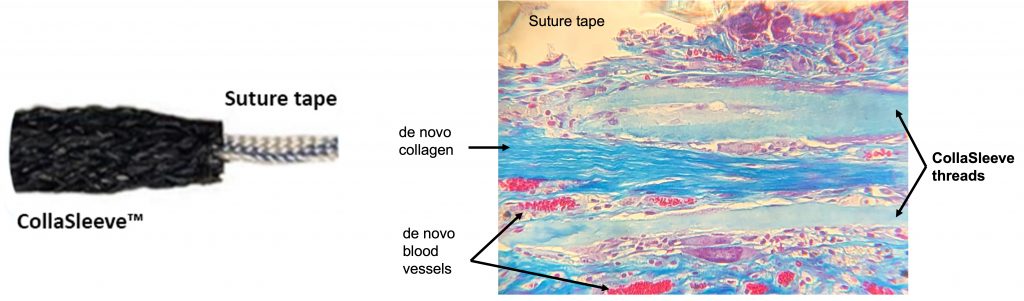
Numerous published in vivo studies demonstrate collagen molecules are unidirectionally aligned within CollaMedix’s ELAC threads, mimicking the native topography of tendon and such topographical mimicry has a tenoinductive effect on mesenchymal stem cells (MSC) by elongating their cytoskeleton. De novo collagen that is deposited over the threads in vivo is also aligned and is positive for the tendon-specific marker tenomodulin, indicating that the fabric of the new collagen made by the body is guided by our ELAC collagen threads and has tendon-like characteristics. This bioinductive property is unique to CollaMedix’s ELAC threads. CollaMedix is developing safer, bioinductive medical implants based on electrocompacted pure collagen threads woven into a biofabric (CollaFabric®). See publications at the end.
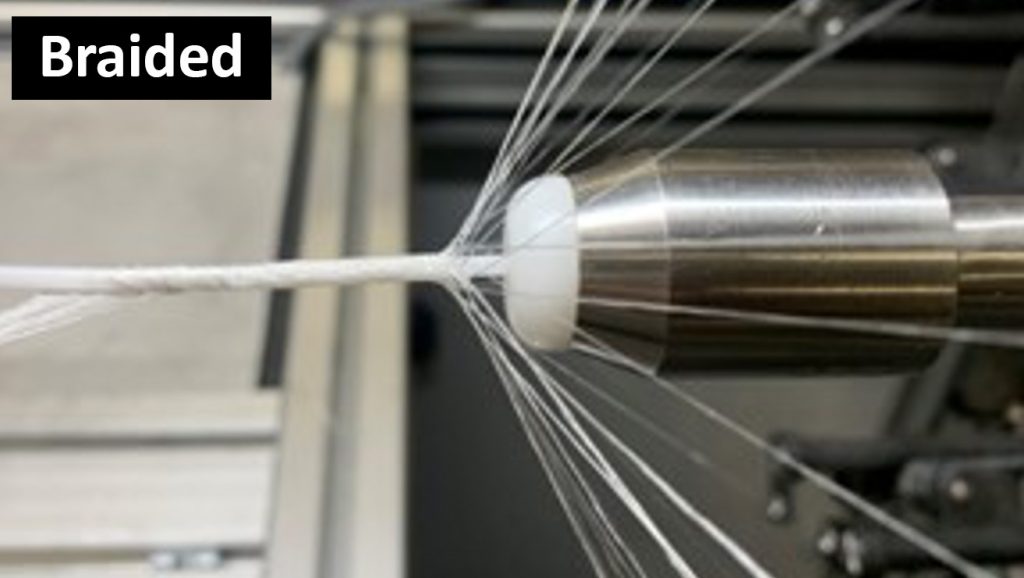
Braiding
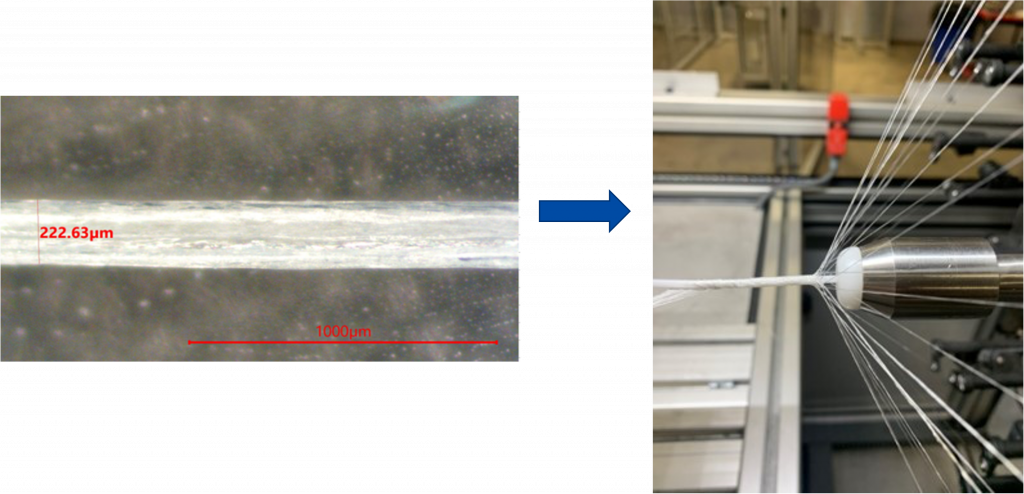
The mechanical robustness of electrocompacted aligned collagen threads made by CollaMedix made it possible to produce our products withstanding the rigors of industrial braiding. This creates the only pure collagen braided tube, scaffold or construct available today. The braiding process endows our products with controllable interconnected macroporosity that facilitates fast and complete cellular infiltration in vivo, allowing for new tissue to form throughout the braid unlike decellularized xenografts such as porcine dermis.
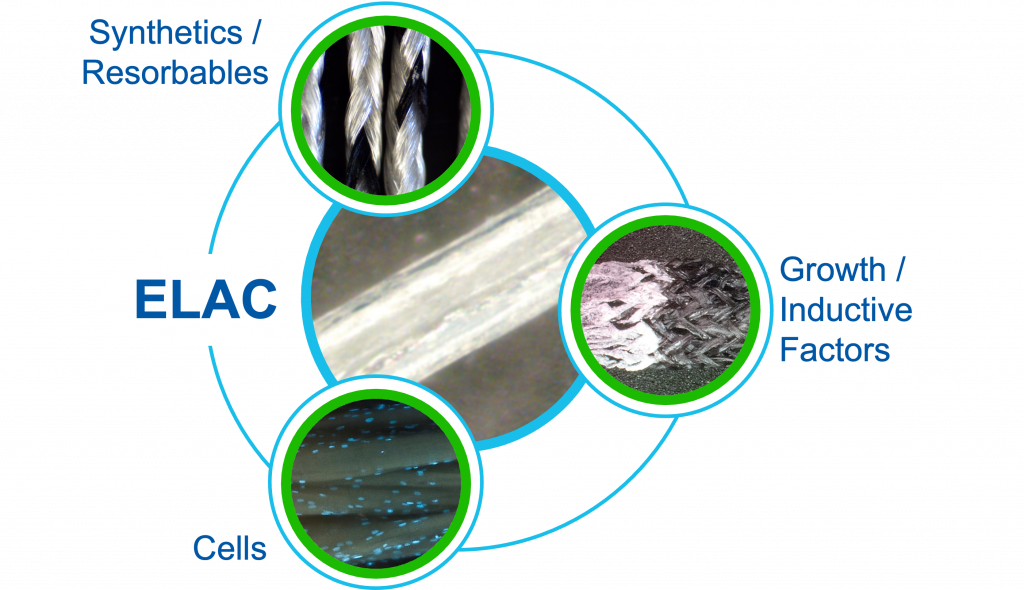
CollaFabric® Hybridized
These highly aligned ELAC collagen threads can be hybridized with other materials to generate specific properties and tissue responses, including, for example:
– Synthetics/Resorbables: UHMW Polyethylene, PLLA, PLGA, etc.
– Cells: Mesenchymal or other stem cells
– Growth/Inductive Factors: Hydroxyapatite for osteoinduction
Platform Technology Pipeline

Products
Products Under Development: Not for Clinical Use. Not Available for Sale in the United States
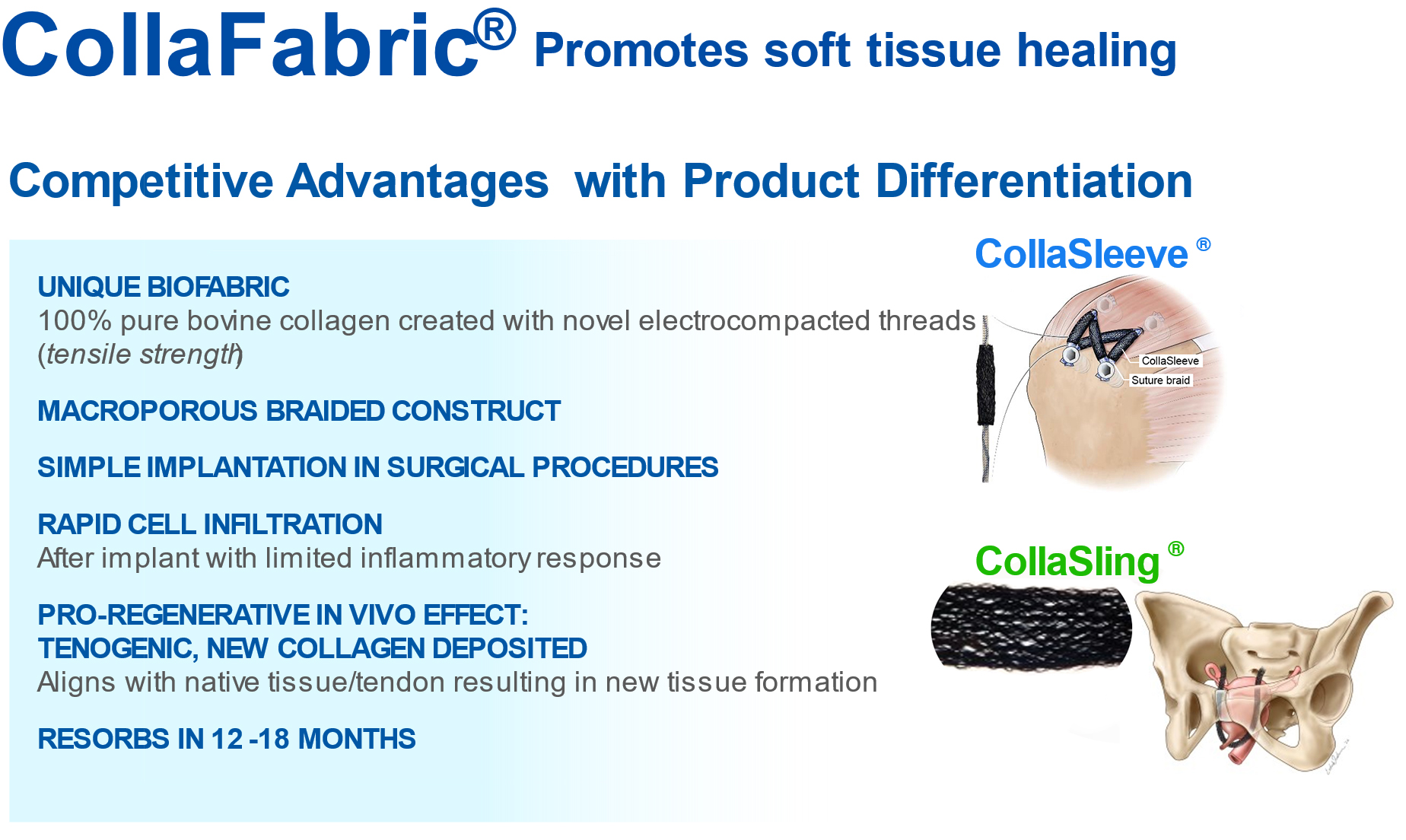
CollaMedix has developed two products to address unmet clinical needs:
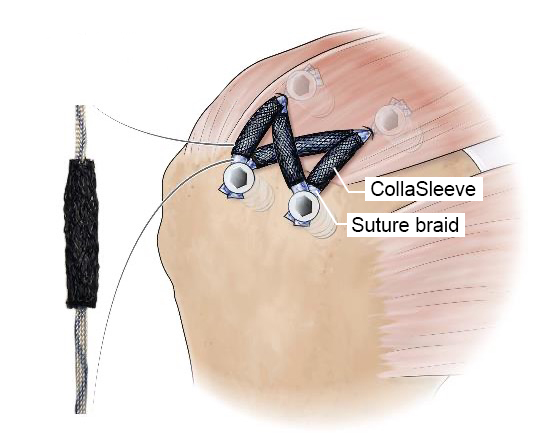
– Orthopedics: CollaSleeve® for treatment of rotator cuff repairs
– Female Health: CollaSling® for stress urinary incontinence in women
Unmet Clinical Need to Reduce Retear Rates in Rotator Cuff Repair
Approximately 500,000 rotator cuff (RC) repair procedures are performed annually. The standard procedure reattaches the tendon to the bone interface by using suture anchors. Certain patients experience high retear rates ranging from 25%-50%-90%.
Failed RC repairs result in costly and complex muscle transfer or arthroplasty procedures. Thus, there is a great interest in regenerative technologies to improve the outcomes of RC repair.
Existing repair patches largely capitalize on decellularized grafts which lack porosity for cellular infiltration and sometimes can be associated with inflammatory rejection. Recently, engineered biologic solutions have emerged containing a collagen matrix with synthetic resorbable materials which are required for strength but may cause an inflammatory response.
CollaSleeve® is a tubular braided biotextile that is deployed simply by sliding over all types of sutures that are used during tendon repairs. This pure collagen macroporous scaffold is implanted using arthroscopic techniques in the standard SpeedBridge RCR procedure. CollaSleeve’s aligned collagen threads cause de novo aligned collagen deposition within the scaffold for rapid tissue in-growth over the tendon to improve the repair. CollaSleeve® improves tissue healing over time by acting as a scaffold that drives organized deposition of a tendon-like matrix at the repair site thereby strengthening the tendon.

Unmet Clinical Need for Safe Products to Treat Stress Urinary Incontinence in Women
CollaSling® is a safer bio-inspired pure collagen sling comprised of a 50cm electrocompacted braided scaffold. Stress Urinary Incontinence (SUI) treatment with synthetic mesh slings, which are also called mid-urethral slings or “tapes”, are placed using a minimally invasive procedure to support the urethra from below like a “hammock”. The figure below shows the anatomical placement of SUI slings. CollaSling® has been shown in several long-term in vivo studies to be highly biocompatible and produce soft tissue repairs with deposition of de novo collagen within the scaffold. Due to its bioinductive and pro-regenerative properties, CollaSling® attracts new collagen with the scaffold creating a new band of strong fibrous tissue for support and repair.


Prototype CollaSling® is the same size as human implants, and has been created and successfully implanted in 14 adult female sheep. The surgeries were performed by Dr. Hijaz, CMO, CollaMedix, who routinely places polypropylene mesh slings in women. He noted that the CollaSling® prototypes were easy to use and were placed quite similarly to the human procedures. Mechanical strength and histopathology from these sheep sacrificed at 3-weeks and 6-months and 12-months post-implant (figure below) indicates excellent tensile strength and connective tissue formation as shown in publication.
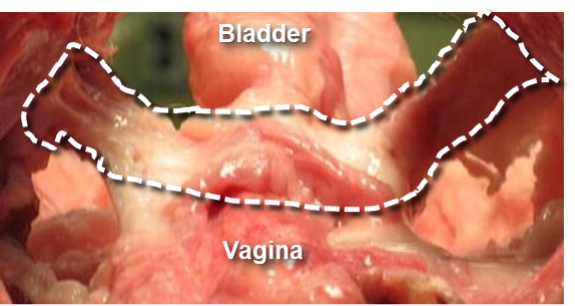
CollaSling® one-year sheep data demonstrated a thick ligamentous band of new collagen between the urethra and vagina
CollaMedix™ Product Features